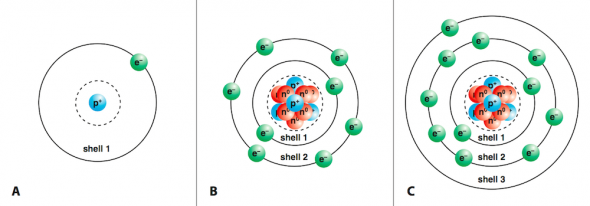
Number of neutrons. Positive ions are small molecules that have gained a positive charge.

Pin By Ayodele Williams On My Saves Ionic Bonding Periodic Table Ionic
Design a positive ion with a charge of 2.

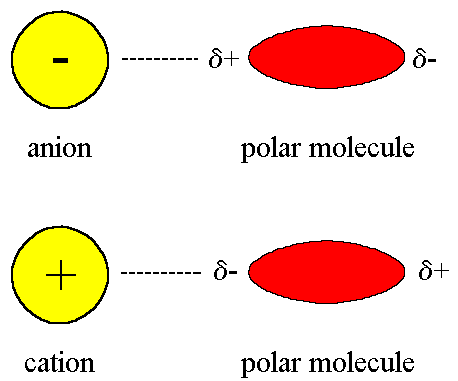
. A positive ion is called a cation and a negative ion is called an anion. Positively charged with a mass of 1 amu and is located in the. Take a 523 µm b 428 µm and θ 549 a Find the magnitude and direction of the resultant force on the electron.
What element is your ion. The center of an atom Proton. And unfortunately in todays world there are significant quantities of positive ions in the air.
An ions charge will be either positive or negative but not both. Nucleus proton neutron electron atom ion charge neutral atomic mass and element. Design a positive ion with a charge of 2 10242011 Moore and Paul from SCIMA 200-03 76 at California College of the Arts.
Try these with your partner. _____ What element is your atom. In order to be considered an ion an atom must have a positive charge.
This charge can be either positive or negative. Chemistry questions and answers. Ionic compounds are electrically.
Metals are electron-rich species that tend to be oxidized. Check your ideas and write down two examples that show your rules work and include a drawing for each. Ions are chemical species that derive from either the electron loss or gain.
3 of electrons. 942019 LoebleinPerkins Page 3 Number of protons _ 4 Number of neutrons__ 5 Number of electrons__ 0 Number of protons. _ Baryllium _____ What element is your atom Helium _____ What mass is your ion.
Let right be the x. Is the nucleus of your ion stable or unstable. What element is your ion.
Monatomic ions are one atom ions example Cl-. The charges are mutually exclusive. 3 of neutrons.
What element is your ion. An electron is near a positive ion of charge 9e and a negative ion of charge 8e see the figure below. Polyatomic ions are two or more atoms grouped together having a net charge example ClO 3-.
2 A positive ion which has positive charge. You see Be Mg Ca Sr Ba. Answers at end of paper 9.
There is nothing you need to record. What is in your atom or ions. Design a positive ion with a charge of 2.
In an ionic compound the total positive charge of all the positive ions. _____ Talk about how the tools in the simulation helped you decide if the atom had. Is it a neutral atom positive ion or negative ion.
5 Number of neutrons_ 2 Number of electrons_ 3 Make the change. Learn ions negative 2 charge with free interactive flashcards. Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing.
There is nothing you need to record. Design a positive ion with a charge of 2 Design a neutral stable atom with a and mass of 9. Most forms of pollution toxic chemicals pollen mold pet dander and other harmful chemicals in the air all carry a positive electrical charge making them positive ions.
These examples should be consistent with the rules you discovered All of your examples should also have a. When ions that have a positive charge form bonds with ions that have a negative charge the charge of the new compound is. Because the Periodic Table reflects electronic structure all of these metals have the TWO VALENCE ELECTRONS which can be lost on oxidation.
Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing. Thus the ion has a positive charge. It loses an electron which means it has more protons than electrons.
Look at the Periodic Table and look at the second COLUMN. Create definitions for the words that list their important aspects and. _____ Show a neutral atom a positive ion and a negative ion.
Write the formula unit of the compound formed from the ions. __ 9_____ What is the charge of you atom___ 0_____ Is the. If an ion has a positive charge then it has lost an electrons.
Draw Your Atom or. The total negative charge of all the negative ions. 1 2 Positive of protons.
Up to 24 cash back ion positive ion one with extra positive charge. 4 0 Neutral 7. _____ What mass is your ion.
Design a positive ion with a charge of 2. 4 of neutrons. Design a neutral stable atom with a mass of 9.
Choose from 500 different sets of ions negative 2 charge flashcards on Quizlet. So you dont gain anything when the ion has a positive charge. What mass is your ion.
Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing. What element is your ion. Therefore these species carry an electrical charge.
2 See answers Advertisement Advertisement Greenleafable Greenleafable The correct answer is charge Any type of charge positive or negative as long as it is a charge. Make sure you know working definitions for. The key difference between positive and negative ion is that the positive ion carries a positive electrical charge whereas the negative ion carries a negative electrical charge.
5 of electrons. Two positive ions each carrying a charge q are kept at a distance d it is found that force of repulsion between them is F d 2 k q q 4 π E 0 1 d 2 q q w h e r e q n e F 4 π E 0 1 d 2 n 2 e 2 n e 2 4 π E 0 F d 2. Draw your atom or ion What is the charge.
The Group 2 metals The Group 2 metals. Apply Ion X has a charge of 2 and ion Y has a charge of 1 -. One type is called cation and the other an anion.
Forces Between Charges. _____ A - ion negative ion one with extra negative charge. _____ 3 A negative ion with negative charge.
Design a positive ion with a charge of 2. There is nothing you need to record. Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing.
Try these with your partner. If it gained an electron then it would have a negative charge. Up to 24 cash back 7.
Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth

5 1 Ionic And Molecular Compounds Introductory Chemistry

What Are Difference Between Atom And Ion Definition Types And Importance Chemistry Aesl

7 3 Lewis Symbols And Structures Chemistry

Ion Read Chemistry Ck 12 Foundation

Atomic Spectrum Of Hydrogen 11th Chemistry Hydrogen Atom Atomic Structure

0 comments
Post a Comment